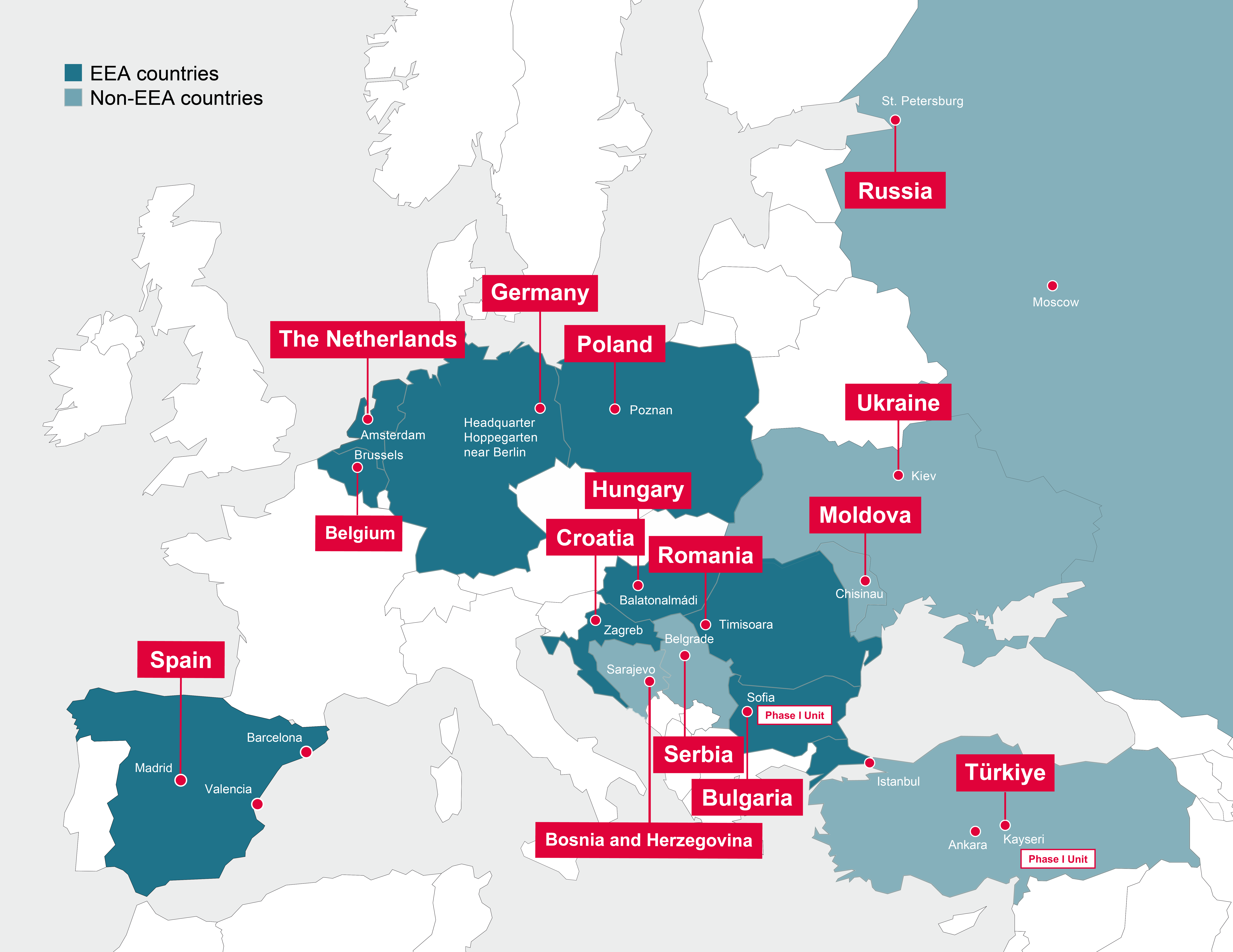
Germany
CCDRD AG Headquarter Hoppegarten, near Berlin
- General Management
- Business Development
- Regulatory and Strategic Advice
- Clinical Operations
- Project Management of phase I-IV trials
- Submission services
- Monitoring of phase II-IV trials in Germany
- Statistics and Data Management
- Quality Managment
- Information Technology
Bulgaria
Sofia
- Management of three local Phase I units
- Site identification and management for phase I-IV trials
- Feasibility evaluation
- Strong clinical networks and access to qualified sites in oncology, CNS, renal disease, diabetes, dermatology, cardiology
- Regulatory submissions
- Specialized monitoring of pharmacokinetic studies
- Monitoring of phase I-IV studies
Croatia
Zagreb
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Strong clinical networks and access to sites in oncology, renal disease, metabolic disease, diabetes, dermatology, ophthalmology
- Regulatory submissions
- Monitoring of phase II/III studies
Hungary
Balatonalmádi
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Strong clinical networks and access to sites in oncology, renal disease, metabolic disease, diabetes, dermatology, ophthalmology
- Regulatory submissions
- Monitoring of phase II/III studies
The Netherlands & Belgium
Amsterdam, Brussels
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Strong clinical networks and access to sites in oncology, renal disease, metabolic disease, diabetes, dermatology, ophthalmology
- Regulatory submissions
- Monitoring of phase II/III studies
Poland
Poznan
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Strong clinical networks and access to sites in oncology, renal disease, metabolic disease, diabetes, dermatology, ophthalmology
- Regulatory submissions
- Monitoring of phase II/III studies
Romania
Timisoara
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Strong clinical networks and access to sites in oncology, renal disease, CNS, diabetes, dermatology
- Regulatory submissions
- Monitoring of phase II/III studies
Spain
Barcelona, Madrid, Valencia
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Strong clinical networks and access to sites in oncology, renal disease, metabolic disease, diabetes, dermatology, ophthalmology
- Regulatory submissions
- Monitoring of phase II/III studies
Bosnia and Herzegovina
Banja Luka
- Feasibility evaluation of clinical sites
- Site identification, contracting and management
- Regulatory submissions
- Monitoring of phase II-III studies
- Enrollment strategy
Moldova
Chisinau
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Strong clinical networks and access to sites in oncology, renal disease, metabolic disease, diabetes, dermatology, ophthalmology
- Regulatory submissions
- Monitoring of phase II/III studies
Russia
Moscow, St. Petersburg
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Strong clinical networks and access to sites in oncology, renal disease, metabolic disease, diabetes, dermatology, ophthalmology
- Regulatory submissions
- Monitoring of phase II/III studies
Serbia
Belgrade
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Regulatory submissions
- Monitoring of phase II-III studies
Türkiye
Kayseri
- Management of local Phase I unit
- Regulatory submissions for phase I trial
- Monitoring of phase I studies
Ukraine
Kiev
- Feasibility evaluation of clinical sites
- Site identification and contracting
- Strong clinical networks and access to sites in oncology, renal disease, metabolic disease, diabetes, dermatology, ophthalmology
- Regulatory submissions
- Monitoring of phase II/III studies