CCDRD AG has performed more than 1000 bioequivalence and bioavailability (BE/BA) trials in healthy volunteers and in patients. Based on this experience, we maintain an inhouse database with pharmacokinetic datasets of more than 500 Active Pharmaceutical Ingredients (API). CCDRD AG runs three phase I units in Bulgaria and one unit in Turkey. All units are positively GCP-inspected by European and other international regulatory authorities. Detailed information about regulatory permissions, timelines, capacity and inspections is provided here.
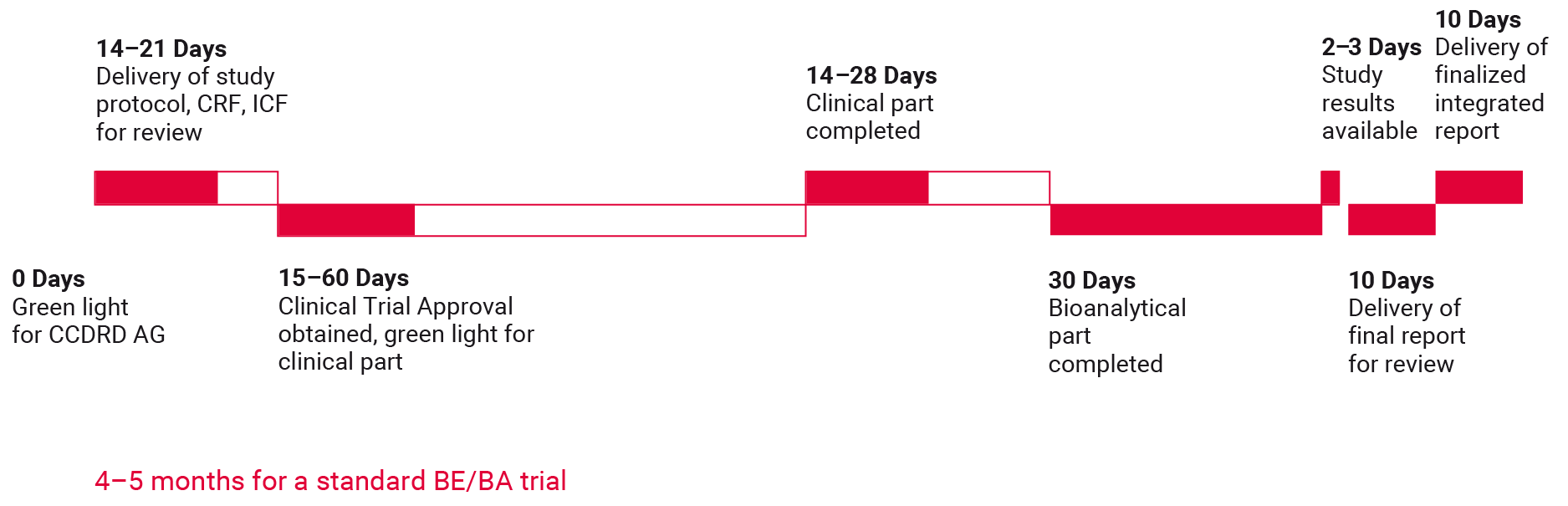
CCDRD AG established a reliable infrastructure for BE/BA trials which takes into account defined procedures and timelines for every step of such trial as well as communication and reporting structures. Our project management team provides detailed timelines for study document writing, submission package preparation, clinical conduct, sample shipment, bioanalyses, data management and statistical analysis up until the delivery date of the final study report.
Services offered:
- Replicate and semi-replicate design
- Crossover design
- Parallel group design
- Two Stage design
- BE/BA trials in healthy subjects and in patients
- Registration packages for Modified-release or Delayed-release products
- Trials with controlled drugs
- Trials with inhalation products
- Trials with topical and transdermal products
Schedule of a bioequivalence study